Home
Our company
We’ve been around more than 10 years
We are a tech company. We offer solutions for clinical research. And we have a product to collect, manage and secure clinical trials data. SCollect.
Our wide expertise in software consulting and custom development allows us to bring technology closer to research projects.
Find how SCollect can help your study succeed
Case studies
These two make us proud
Blog
Our news and views
Customization of the Dashboard trough interactive graphics
From SCollect we continue to make the day to day of the professionals a little easier, the latest SCollect update allows data …
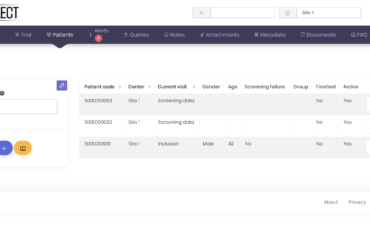
Patients list view
You can now change the patients list view clicking on the button Visits window view, this will allow you to see a …